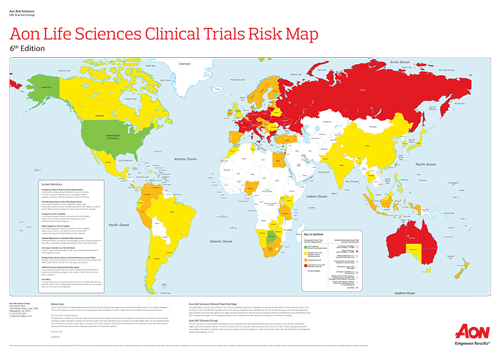
The globalization of clinical trials continues to rise, leading to a plethora of regulatory and operational challenges for the Life Sciences industry. Aon's latest Clinical Trials Risk Map provides an overview of the global clinical trials risk landscape in more than 80 countries, highlighting potential challenges for life sciences companies that conduct global clinical trials.
Download Aon's Life Sciences Clinical Trials Risk Map
James Walters
Managing Director & Life Sciences Practice Leader